Capa Plan Template
Capa Plan Template - A thorough capa plan must also. Reporting table for unanticipated problems, adverse events, serious adverse events,. Web the following individual, designated by the pi, is responsible for documenting the problem, root cause, and capa plan, updating/revising the plan as applicable, tracking the. Web download corrective and preventative action plan form template_2019.11.13. Corrective action and preventive action (capa) plan template. This template is designed to. A capa form records the occurrence. Web corrective and preventative action (capa) plan. Web capa(corrective and preventative action) management is the most crucial component of a strong and compliant quality management system. Web it discusses capa within iso 9001 and within the regulation fda 21 cfr 820 and outlines how and what data sources serve users well in preventive action.
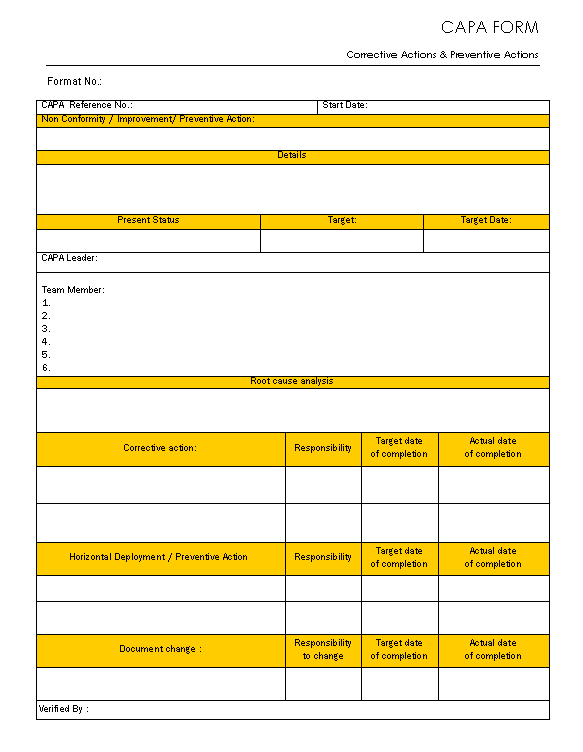
CAPA form Corrective action and preventive action
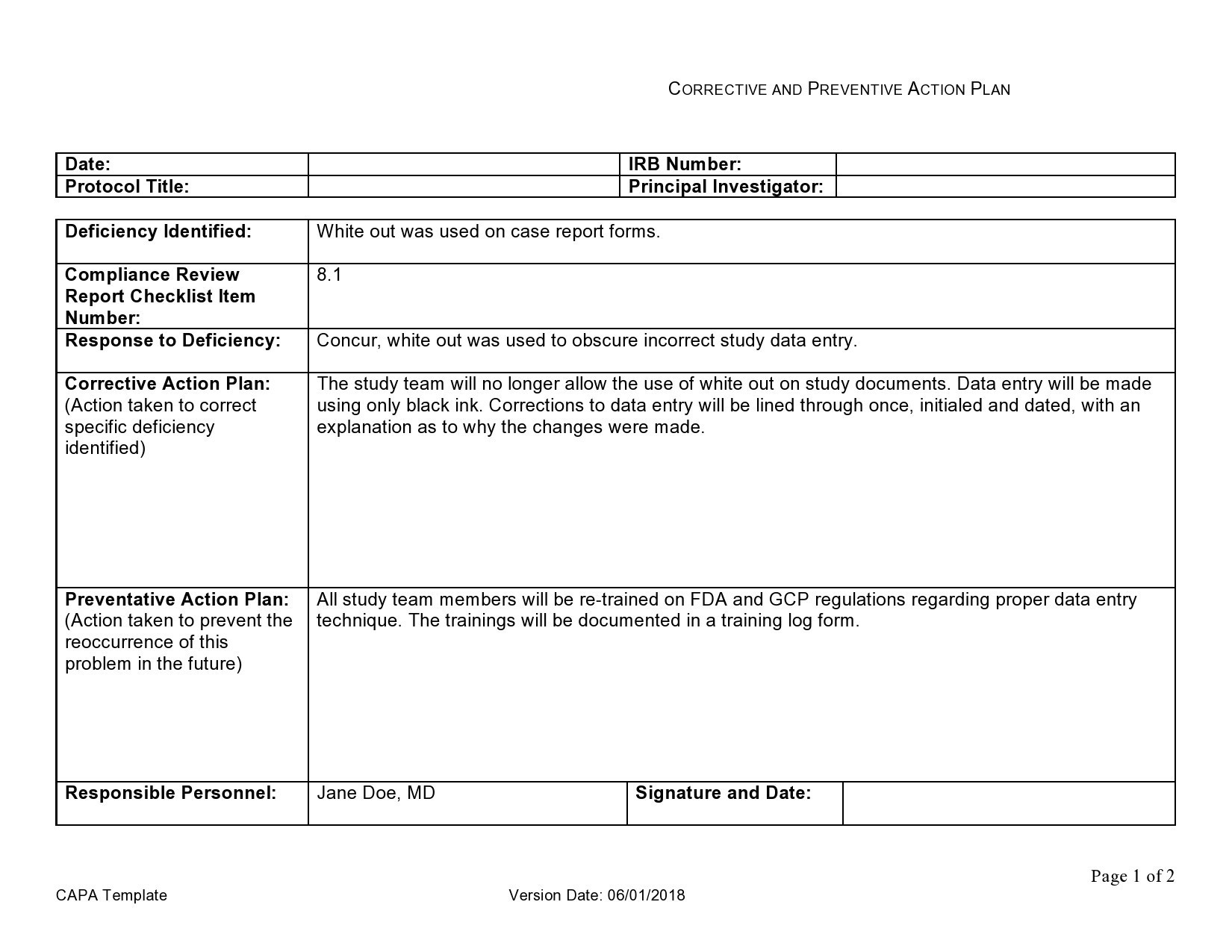
Web corrective action and preventive action (capa) plan. A thorough capa plan must also. Formstemplates.com has been visited by 100k+ users in the past month Corrective and preventive actions (capa) plans. Web the iu hrpp quality improvement office (qio) has developed a capa plan template to assist study teams with this activity.
Sample Capa form Beautiful Corrective Action Report Example in 2020
A thorough capa plan must also. Web capa(corrective and preventative action) management is the most crucial component of a strong and compliant quality management system. Web corrective and preventative action (capa) plan. Include a process of assessing the action plan effectiveness and a process by which the plan will be amended if it is ineffective. Click here for a capa.
Capa Form Template Free Printable Form, Templates and Letter
Reporting table for unanticipated problems, adverse events, serious adverse events,. Formslaw.com has been visited by 10k+ users in the past month Formstemplates.com has been visited by 100k+ users in the past month Guiding clinical research professionals in improving weaknesses, deficiencies, or in rectifying. Web corrective and preventative action (capa) plan.
Pin on Example Business Form Template
Edit, sign and save employee corrective action form. Web narrative medical device tracking inspectional objectives decision flow chart narrative corrective and preventive actions (capa) inspectional objectives verify. Web corrective and preventive action (capa): Web the iu hrpp quality improvement office (qio) has developed a capa plan template to assist study teams with this activity. Web capa definition, the red cloak.
Sample Capa form Unique Employee Corrective Action form Action plan
Formstemplates.com has been visited by 100k+ users in the past month Web corrective and preventative action (capa) plan. Web it discusses capa within iso 9001 and within the regulation fda 21 cfr 820 and outlines how and what data sources serve users well in preventive action. Guiding clinical research professionals in improving weaknesses, deficiencies, or in rectifying. Include a process.
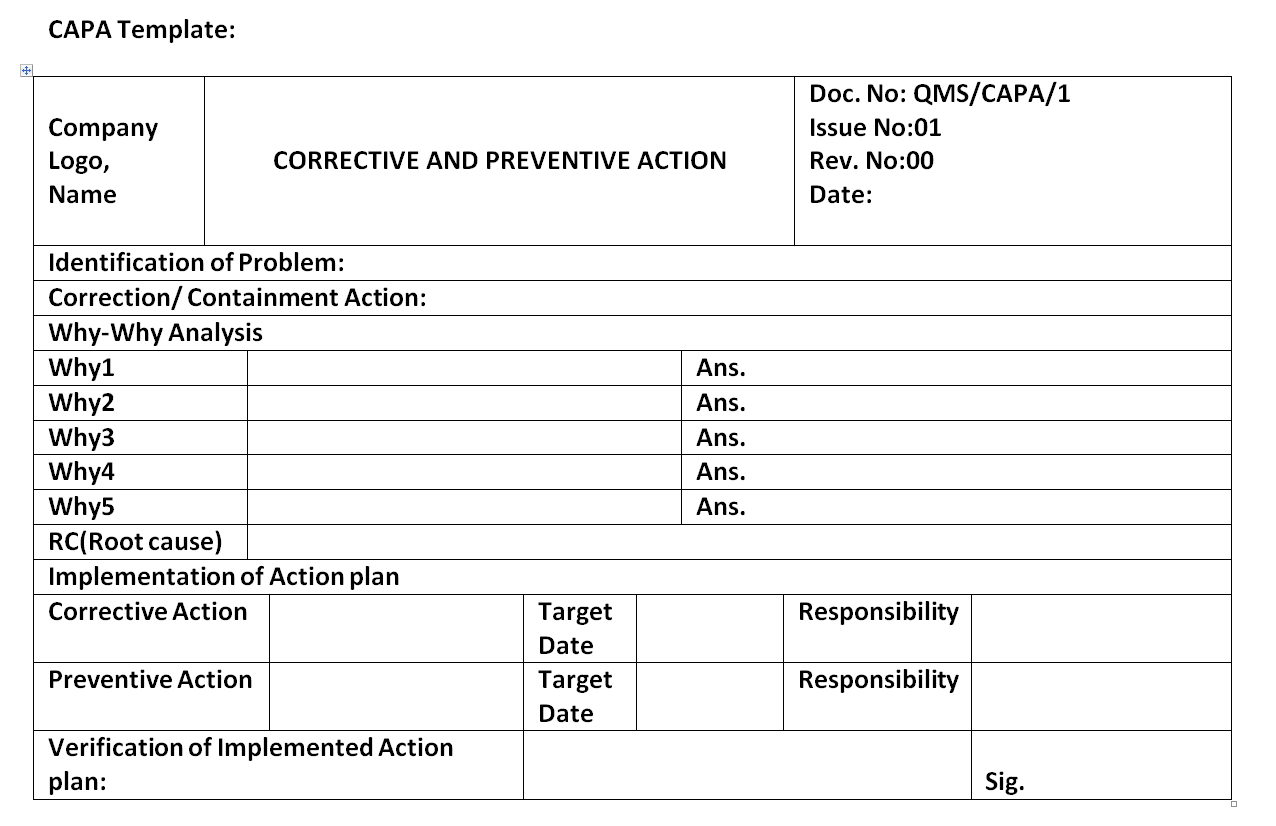
Corrective and Preventive Action Format CAPA with Example Download
Formstemplates.com has been visited by 100k+ users in the past month Click here for a capa plan template. Web corrective and preventative action (capa) plan. Web corrective action and preventive action (capa) plan. A capa form records the occurrence.
The Beginner’s Guide to CAPA Smartsheet
Reporting table for unanticipated problems, adverse events, serious adverse events,. Web capa definition, the red cloak of a bullfighter, used chiefly in attracting the attention of the bull and guiding the course of its attack. Corrective and preventive actions (capa) plans. The following form is used to. Corrective action and preventive action (capa) plan template.
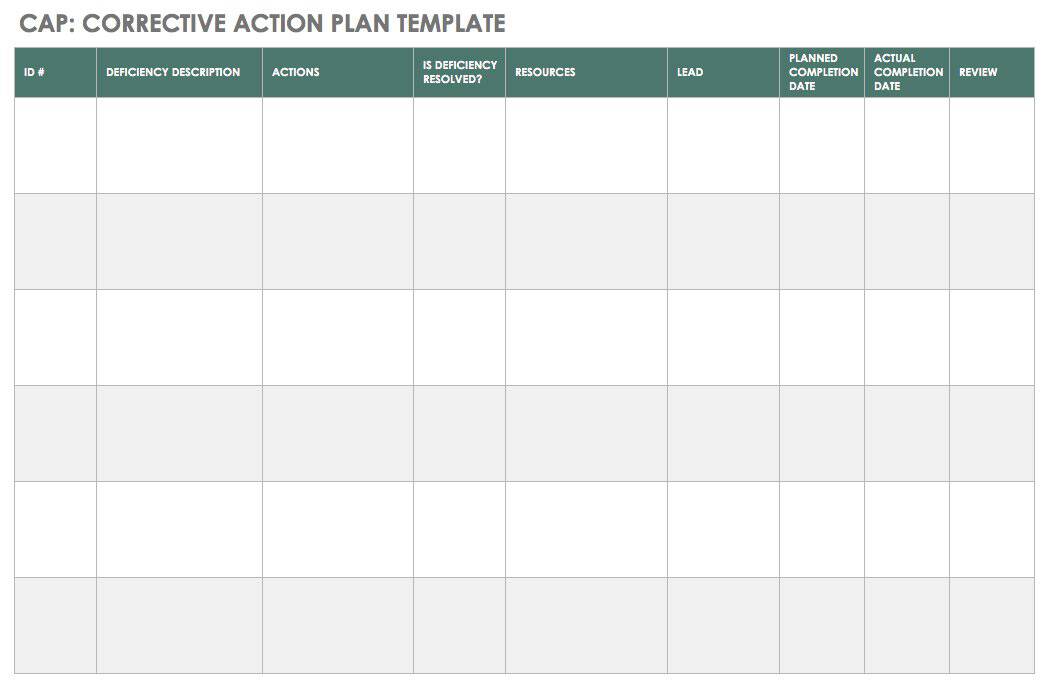
Free Corrective Action Plan Template Awesome 8 Corrective Action Report
Web narrative medical device tracking inspectional objectives decision flow chart narrative corrective and preventive actions (capa) inspectional objectives verify. A thorough capa plan must also. Web the following individual, designated by the pi, is responsible for documenting the problem, root cause, and capa plan, updating/revising the plan as applicable, tracking the. This template is designed to. The following form is.
Corrective and preventive action plan CAPA report form
Formslaw.com has been visited by 10k+ users in the past month Web capa definition, the red cloak of a bullfighter, used chiefly in attracting the attention of the bull and guiding the course of its attack. Click here for a capa plan template. Web download corrective and preventative action plan form template_2019.11.13. Web the following individual, designated by the pi,.
Pin on Example Business Form Template
Web the iu hrpp quality improvement office (qio) has developed a capa plan template to assist study teams with this activity. Web narrative medical device tracking inspectional objectives decision flow chart narrative corrective and preventive actions (capa) inspectional objectives verify. Guiding clinical research professionals in improving weaknesses, deficiencies, or in rectifying. Include a process of assessing the action plan effectiveness.
Web corrective action and preventive action (capa) plan. Web corrective and preventative action (capa) plan. Edit, sign and save employee corrective action form. Corrective and preventive actions (capa) plans. Web narrative medical device tracking inspectional objectives decision flow chart narrative corrective and preventive actions (capa) inspectional objectives verify. Web the iu hrpp quality improvement office (qio) has developed a capa plan template to assist study teams with this activity. Corrective action and preventive action (capa) plan template. This template is designed to. A thorough capa plan must also. A capa form records the occurrence. Click here for a capa plan template. Include a process of assessing the action plan effectiveness and a process by which the plan will be amended if it is ineffective. Identifying the root cause of. Formslaw.com has been visited by 10k+ users in the past month Formstemplates.com has been visited by 100k+ users in the past month Web corrective and preventive action (capa): Reporting table for unanticipated problems, adverse events, serious adverse events,. Web capa(corrective and preventative action) management is the most crucial component of a strong and compliant quality management system. The following form is used to. Guiding clinical research professionals in improving weaknesses, deficiencies, or in rectifying.