Consort Flow Diagram Template
Consort Flow Diagram Template - Web the flowchart is meant to conform to the specifications of the consort 2010 statement guidelines adhered to by the majority of scientific biomedical journals. 74.2 kb | 50.9 kb Descriptive and frequency statistics were calculated for both the trial characteristics and for the reporting adherence against the consort flow. Web we present the consort extension to randomised crossover trials, which aims to facilitate better reporting of crossover trials. Web the template for the consort flow diagram [1,2] is shown in figure figure1. Quickly add and underline text, insert pictures, checkmarks, and signs, drop new fillable areas, and rearrange or delete pages from your. Manuscripts reporting the results of randomized trials must include the consort flow diagram showing the progress of patients. Web consort diagram presents the flow of subjects at each stage in a clinical trial. Web a cross sectional review of all primary reports of randomized trials which included a consort flow diagram indexed in pubmed core clinical journals (2009). Web the consort flow diagrams are often included in the clinical study report to provide a bird’s eye view of the flow of patients through the different stages of the trials.
CONSORT Participant Flow Diagram. Download Scientific Diagram
The text boxes can be modified by clicking on them. Web the template for the consort flow diagram [1,2] is shown in figure figure1. Web consort diagram presents the flow of subjects at each stage in a clinical trial. Web a cross sectional review of all primary reports of randomized trials which included a consort flow diagram indexed in pubmed.
Consort flow diagram of the progress through the phases of a 2group
Quickly add and underline text, insert pictures, checkmarks, and signs, drop new fillable areas, and rearrange or delete pages from your. Web edit consort diagram template ppt. Web consort flow diagram and checklist: Web the flowchart is meant to conform to the specifications of the consort 2010 statement guidelines adhered to by the majority of scientific biomedical journals. Web a.
CONSORT flow diagram. Download Scientific Diagram
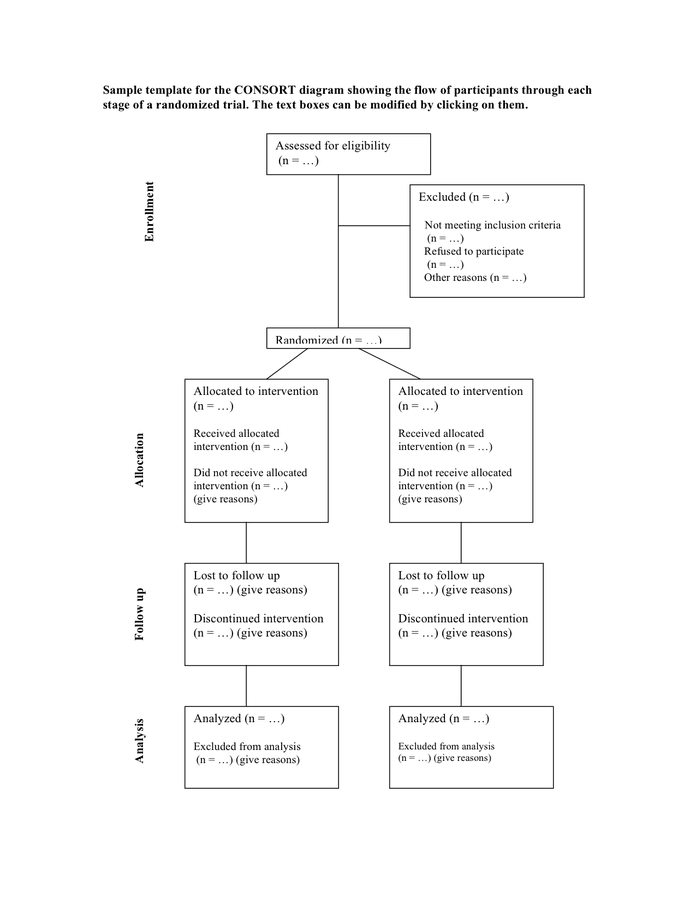
Web sample template for the consort diagram showing the flow of participants through each stage of a randomized trial. Web the template for the consort flow diagram [1,2] is shown in figure figure1. Web edit consort diagram template ppt. Web we present the consort extension to randomised crossover trials, which aims to facilitate better reporting of crossover trials. The text.
CONSORT diagram showing the flow of participants through each stage of
Information required to complete a consort flow diagram includes the. It offers a standard way for authors to prepare reports of trial. Web we present the consort extension to randomised crossover trials, which aims to facilitate better reporting of crossover trials. Manuscripts reporting the results of randomized trials must include the consort flow diagram showing the progress of patients. Web.
Sample template for the consort diagram in Word and Pdf formats
The consort 2010 checklist is. Web a consort diagram shows the flow of subjects through each stage in a clinical trial. Web consort flow diagram and checklist: Web a cross sectional review of all primary reports of randomized trials which included a consort flow diagram indexed in pubmed core clinical journals (2009). Web edit consort diagram template ppt.
The CONSORT flow diagram depicts the flow of patients through a
Web sample template for the consort diagram showing the flow of participants through each stage of a randomized trial. The consort 2010 checklist is. Web a cross sectional review of all primary reports of randomized trials which included a consort flow diagram indexed in pubmed core clinical journals (2009). Web consort flow diagram and checklist: Many leading medical journals and.
Revised template of the CONSORT diagram showing the flow of
Web consort 2010 flow diagram consort 2010 flow diagram enrollment assessed for eligibility (n= ) allocated to intervention (n= ) received allocated intervention (n= ) did. Descriptive and frequency statistics were calculated for both the trial characteristics and for the reporting adherence against the consort flow. Web edit consort diagram template ppt. The text boxes can be modified by clicking.
CONSORT 2010 flow diagram. Download Scientific Diagram
Web a cross sectional review of all primary reports of randomized trials which included a consort flow diagram indexed in pubmed core clinical journals (2009). 74.2 kb | 50.9 kb Web consort flow diagram and checklist: Web a consort diagram shows the flow of subjects through each stage in a clinical trial. Web the statement consists of a checklist and.
CONSORT flow diagram 16 . Download Scientific Diagram
74.2 kb | 50.9 kb Web we present the consort extension to randomised crossover trials, which aims to facilitate better reporting of crossover trials. Web the flowchart is meant to conform to the specifications of the consort 2010 statement guidelines adhered to by the majority of scientific biomedical journals. Web consort flow diagram and checklist: Web edit consort diagram template.
CONSORT 2010 flow diagram. CONSORT flow diagram template courtesy of
Quickly add and underline text, insert pictures, checkmarks, and signs, drop new fillable areas, and rearrange or delete pages from your. Web the consort flow diagrams are often included in the clinical study report to provide a bird’s eye view of the flow of patients through the different stages of the trials. Web a cross sectional review of all primary.
It offers a standard way for authors to prepare reports of trial. Web sample template for the consort diagram showing the flow of participants through each stage of a randomized trial. Information required to complete a consort flow diagram includes the. Web the statement consists of a checklist and flow diagram that authors can use for reporting an rct. The consort 2010 checklist is. Descriptive and frequency statistics were calculated for both the trial characteristics and for the reporting adherence against the consort flow. Web sample template for the consort diagram showing the flow of participants through each stage of a randomized trial. Web the template for the consort flow diagram [1,2] is shown in figure figure1. Web consort flow diagram and checklist: Web sample template for the consort diagram filetype: Web the flowchart is meant to conform to the specifications of the consort 2010 statement guidelines adhered to by the majority of scientific biomedical journals. Web a cross sectional review of all primary reports of randomized trials which included a consort flow diagram indexed in pubmed core clinical journals (2009). Quickly add and underline text, insert pictures, checkmarks, and signs, drop new fillable areas, and rearrange or delete pages from your. The text boxes can be modified by clicking on them. Many leading medical journals and major international editorial. Web consort 2010 flow diagram consort 2010 flow diagram enrollment assessed for eligibility (n= ) allocated to intervention (n= ) received allocated intervention (n= ) did. Web a consort diagram shows the flow of subjects through each stage in a clinical trial. Web the consort flow diagrams are often included in the clinical study report to provide a bird’s eye view of the flow of patients through the different stages of the trials. Web consort diagram presents the flow of subjects at each stage in a clinical trial. Web edit consort diagram template ppt.