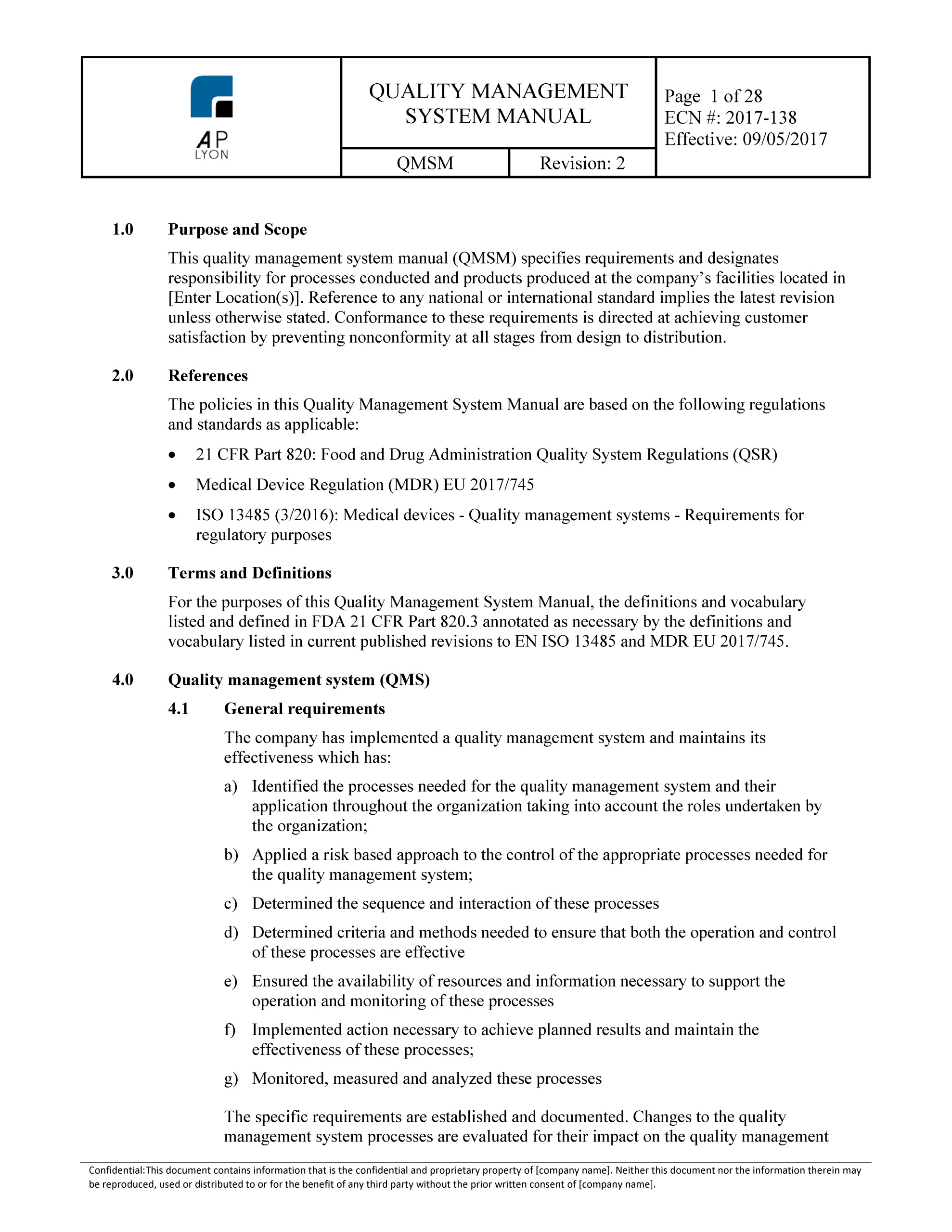
Iso 13485 Templates
Iso 13485 Templates - Web medical device quality system templates. Web take a sneak peek at all the documentation templates by downloading this iso 13485 & eu mdr free demo, and get a closer look at, e.g., how to define clear policies to qualify. Web get latest iso 13485 templates for medical device from i3cglobal. Checklist of mandatory documentation, description of requirements, implementation diagram, etc. Here are all our posts on this standard, and also all questions our consulting. Web the iso 13485 is the standard for quality management in the medical device industry. Web this checklist is completed every time an initial supplier evaluation is carried out or if a blocked supplier is evaluated again for approval. This table maps all requirements of the iso 13485:2016 (by section) to the relevant documents. Here you can check the complete list of documentation, procedure and sop. Web templates iso 13485 templates updated may 24, 2023 template:
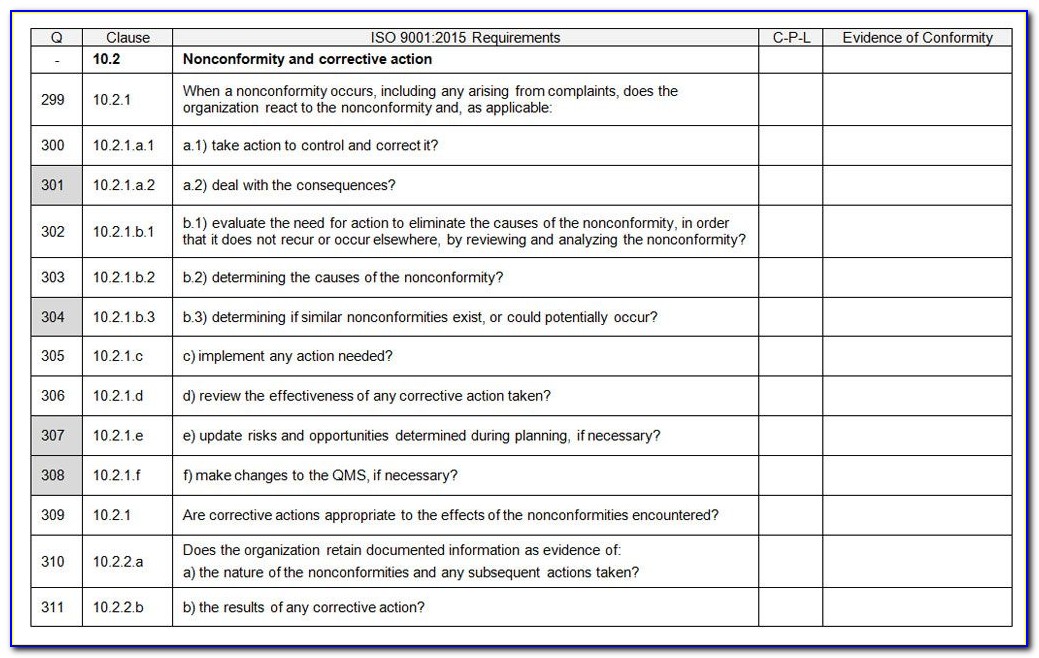
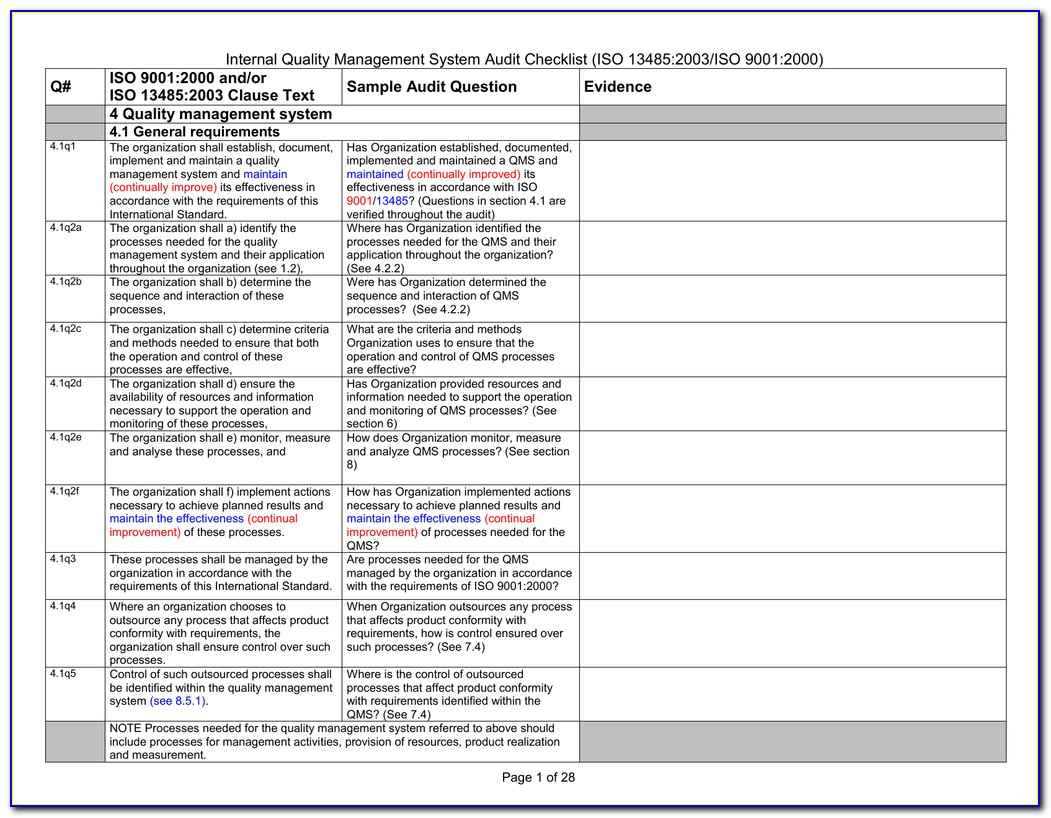
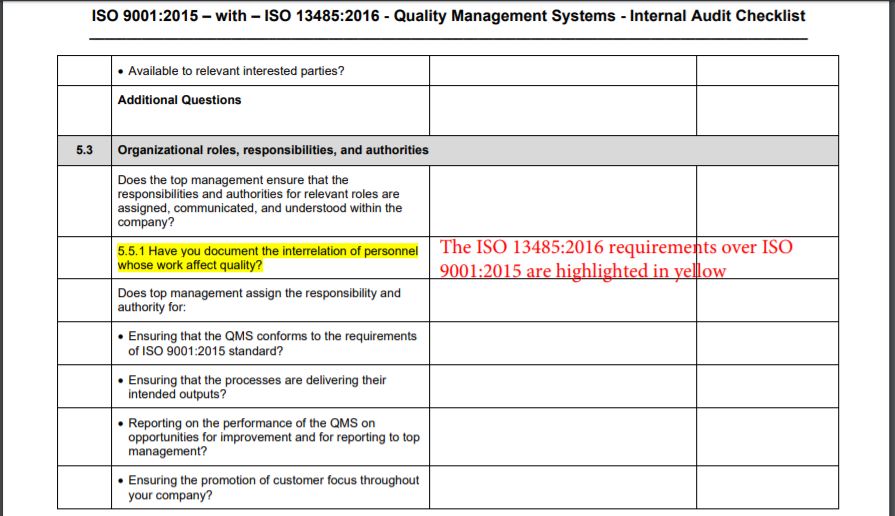
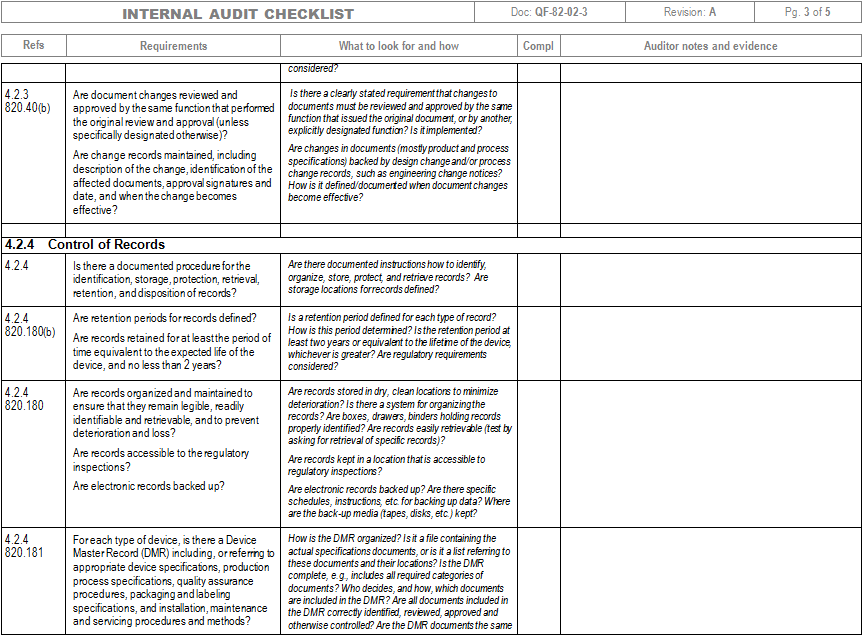
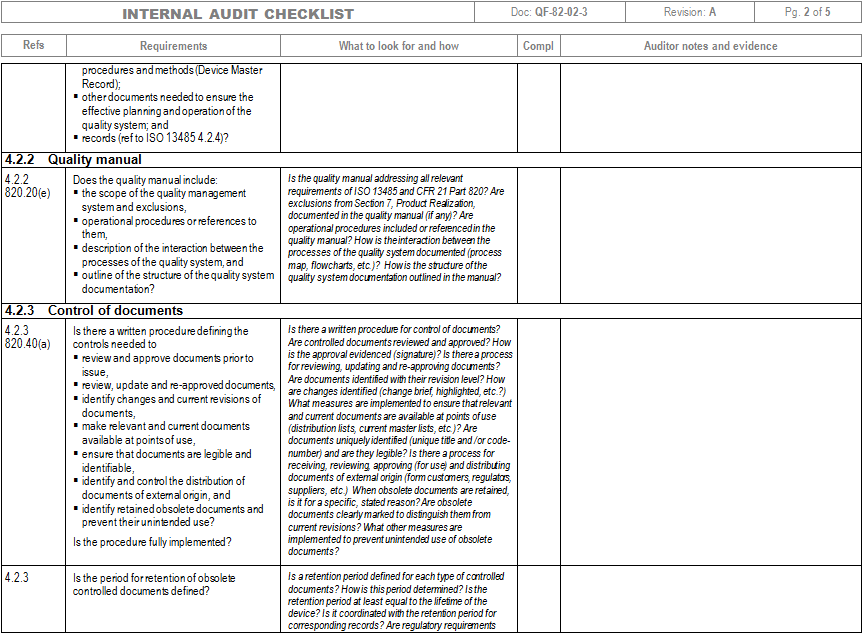
Iso 13485 internal audit checklist advfaher
Web the iso 13485 is the standard for quality management in the medical device industry. Web get latest iso 13485 templates for medical device from i3cglobal. Here are all our posts on this standard, and also all questions our consulting. Web take a sneak peek at all the documentation templates by downloading this iso 13485 & eu mdr free demo,.
Internal Audit Plan Template Iso 13485 Templates Mta1otk1 Resume Gambaran
Web in accordance with both the fda qsr and the iso13485 law, before you can implement a qms system, including a form system, you have to validate it for intended use. For iso 13485, iec 62304, iso 14971 and iec 62366. Web iso 13485:2016 mapping of requirements to documents. Download them for free and get your. Here are all our.
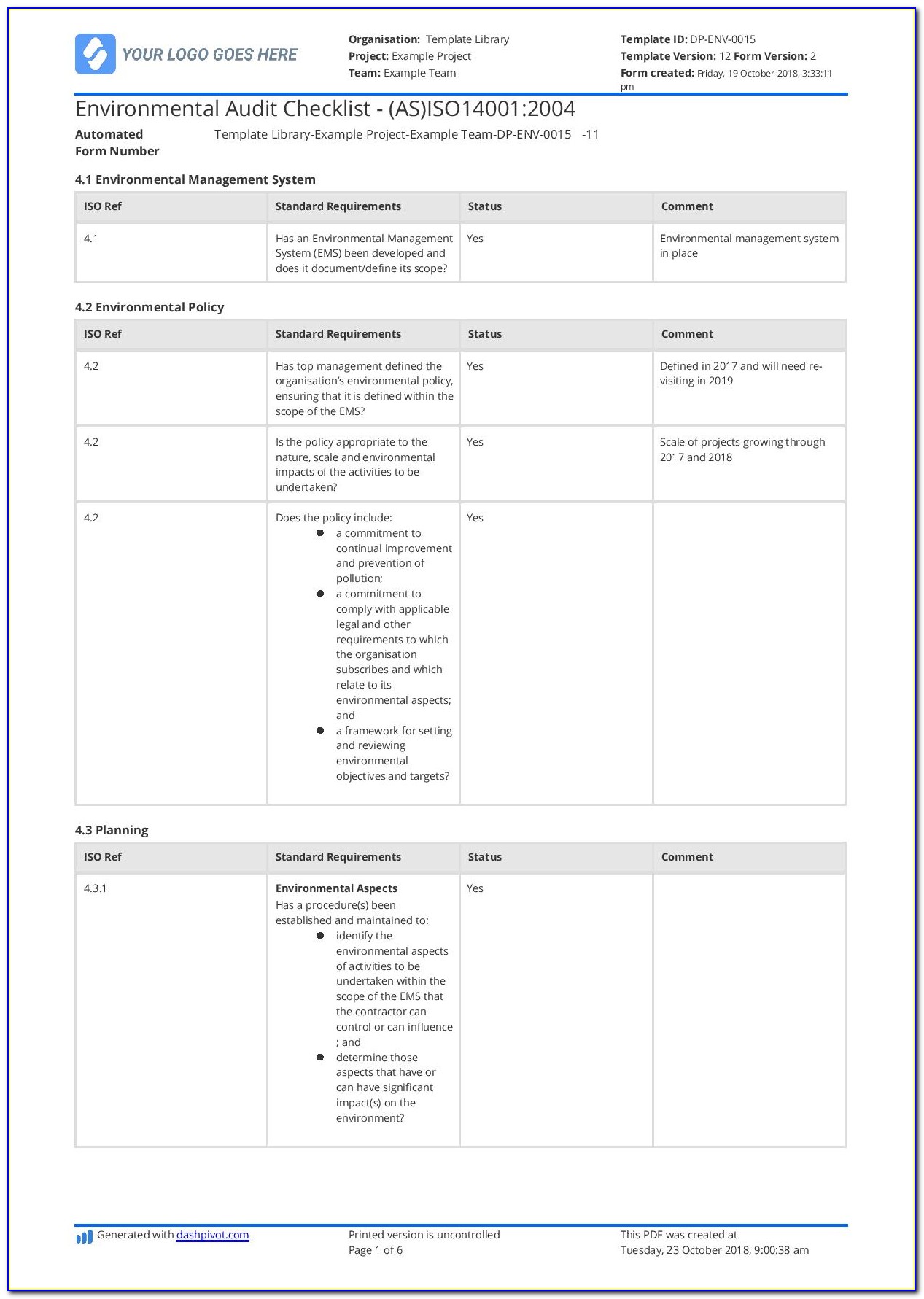
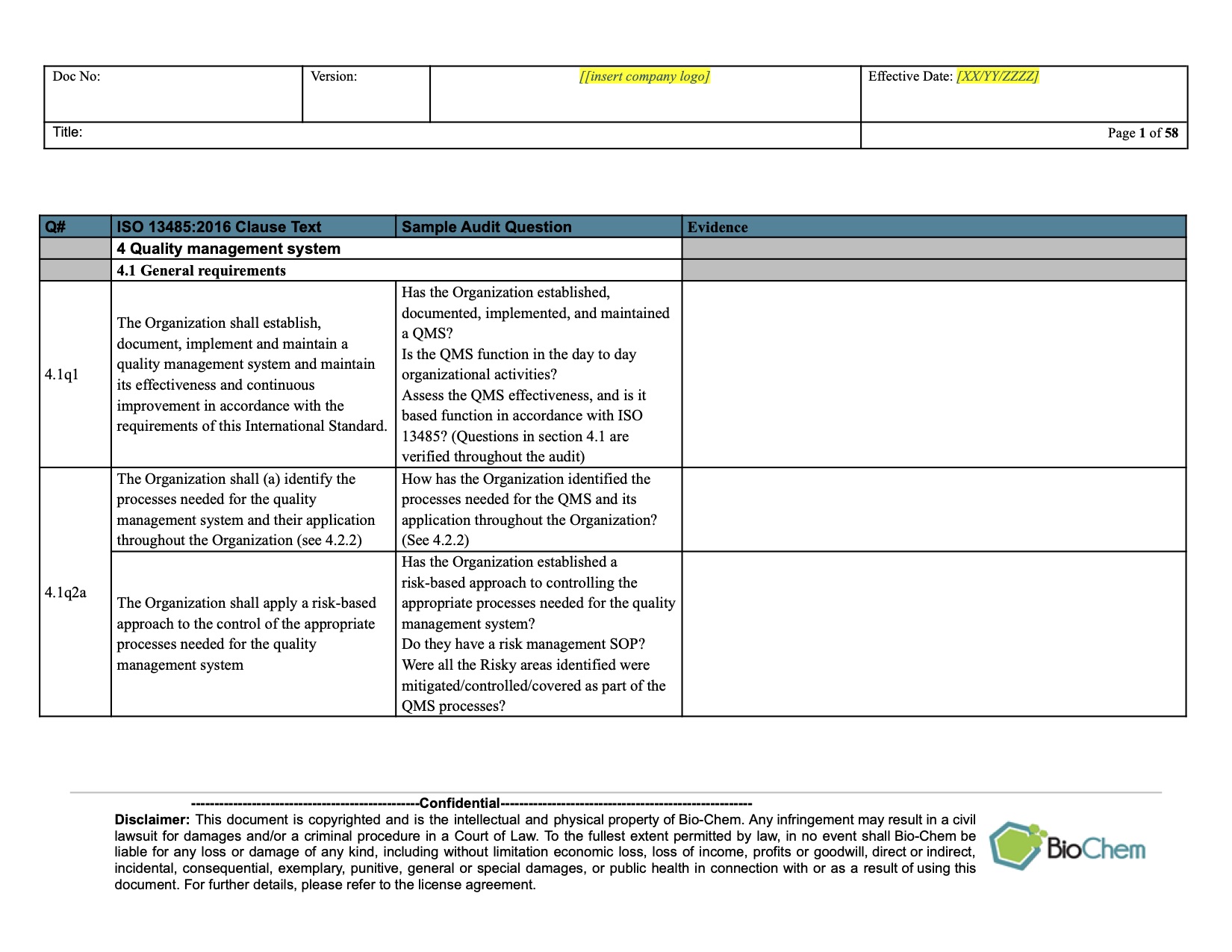
Iso 13485 Audit Report Template
Web design and development plan template (medical device per iso 13485 and 21 cfr 820) free. Web in this article, you will find a quality manual template conforming to the requirements of regulation 2017/745 and en iso 13485:2016 + a11:2021. This table maps all requirements of the iso 13485:2016 (by section) to the relevant documents. Web templates iso 13485 templates.
Iso 13485 internal audit checklist free nestluli
Web get latest iso 13485 templates for medical device from i3cglobal. Web take a sneak peek at all the documentation templates by downloading this iso 13485 & eu mdr free demo, and get a closer look at, e.g., how to define clear policies to qualify. Web in accordance with both the fda qsr and the iso13485 law, before you can.
Best Tips ISO 13485 procedures with our free template (Version 2016)
Web templates iso 13485 templates updated may 24, 2023 template: The documentation template may be used for iso 13485 certification audit purposes. Download them for free and get your. Document templates contain an average of twenty comments each,. Web the documentation template may be used for iso 13485 certification audit purposes.
Iso 13485 Internal Audit Checklist
Gst/vat) this template will provide you with a framework to complete. Med dev qms provides iso 13485:2016 and fda qsr compliant quality system templates specifically developed for startup & small. Web take a sneak peek at all the documentation templates by downloading this iso 13485 & eu mdr free demo, and get a closer look at, e.g., how to define.
Iso 13485 Internal Audit Checklist
Web iso 13485:2016 mapping of requirements to documents. Web design and development plan template (medical device per iso 13485 and 21 cfr 820) free. This table maps all requirements of the iso 13485:2016 (by section) to the relevant documents. Quality manual, policy, objectives dr. Web take a sneak peek at all the documentation templates by downloading this iso 13485 &.
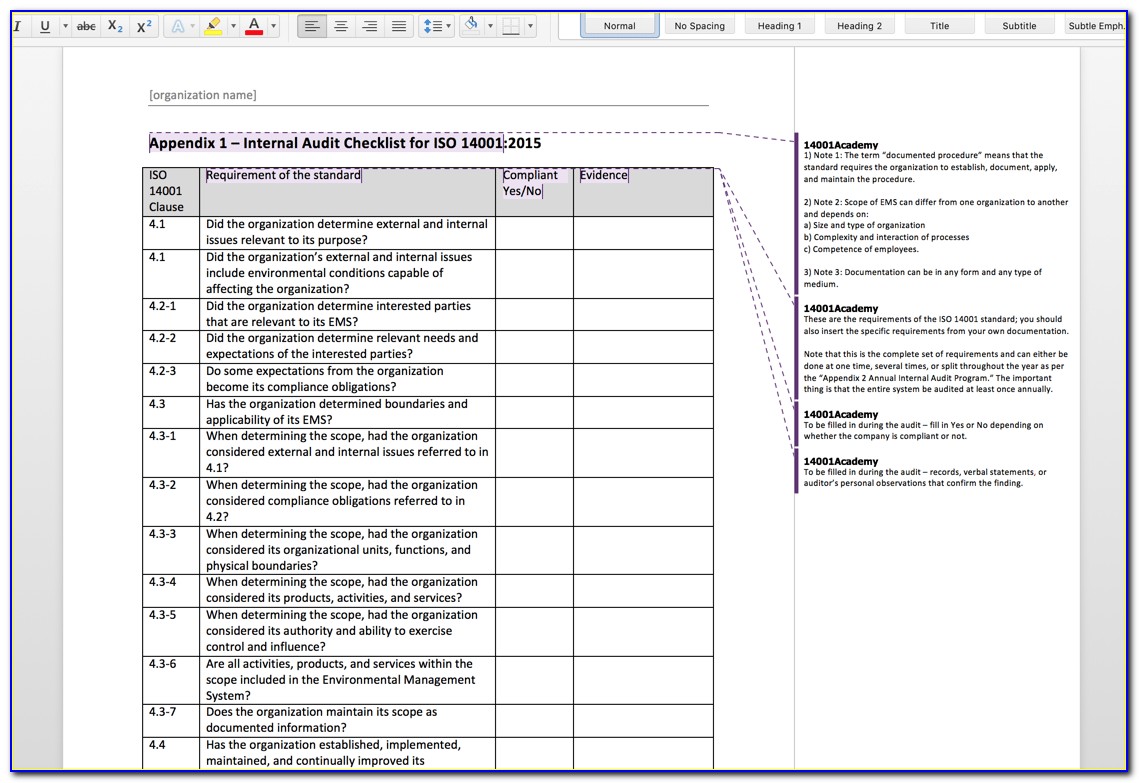
Iso 13485 Internal Audit Schedule Template Gambaran
Web the iso 13485 is the standard for quality management in the medical device industry. This table maps all requirements of the iso 13485:2016 (by section) to the relevant documents. Web in accordance with both the fda qsr and the iso13485 law, before you can implement a qms system, including a form system, you have to validate it for intended.
ISO 13485 Internal Audit Planning and Scheduling BioChem
Web medical device quality system templates. For iso 13485, iec 62304, iso 14971 and iec 62366. Oliver eidel template download this is a free template, provided. The documentation template may be used for iso 13485 certification audit purposes. Download them for free and get your.
Iso 13485 Quality Manual Template Free
Web get latest iso 13485 templates for medical device from i3cglobal. Web simply put, iso 13485 is a set of requirements defined by the international organization for standardization, designed to be used by medical device manufacturers. This table maps all requirements of the iso 13485:2016 (by section) to the relevant documents. Web this checklist is completed every time an initial.
Web design and development plan template (medical device per iso 13485 and 21 cfr 820) free. Document templates contain an average of twenty comments each,. Download them for free and get your. Web the iso 13485 is the standard for quality management in the medical device industry. Here are all our posts on this standard, and also all questions our consulting. Med dev qms provides iso 13485:2016 and fda qsr compliant quality system templates specifically developed for startup & small. Gst/vat) this template will provide you with a framework to complete. Web iso 13485:2016 mapping of requirements to documents. Web get latest iso 13485 templates for medical device from i3cglobal. Web take a sneak peek at all the documentation templates by downloading this iso 13485 & eu mdr free demo, and get a closer look at, e.g., how to define clear policies to qualify. Web in accordance with both the fda qsr and the iso13485 law, before you can implement a qms system, including a form system, you have to validate it for intended use. This table maps all requirements of the iso 13485:2016 (by section) to the relevant documents. Checklist of mandatory documentation, description of requirements, implementation diagram, etc. Quality manual, policy, objectives dr. Oliver eidel template download this is a free template, provided. The documentation template may be used for iso 13485 certification audit purposes. Internal audit plan sven piechottka template download this is a free template, provided by. For iso 13485, iec 62304, iso 14971 and iec 62366. Web in this article, you will find a quality manual template conforming to the requirements of regulation 2017/745 and en iso 13485:2016 + a11:2021. Web simply put, iso 13485 is a set of requirements defined by the international organization for standardization, designed to be used by medical device manufacturers.