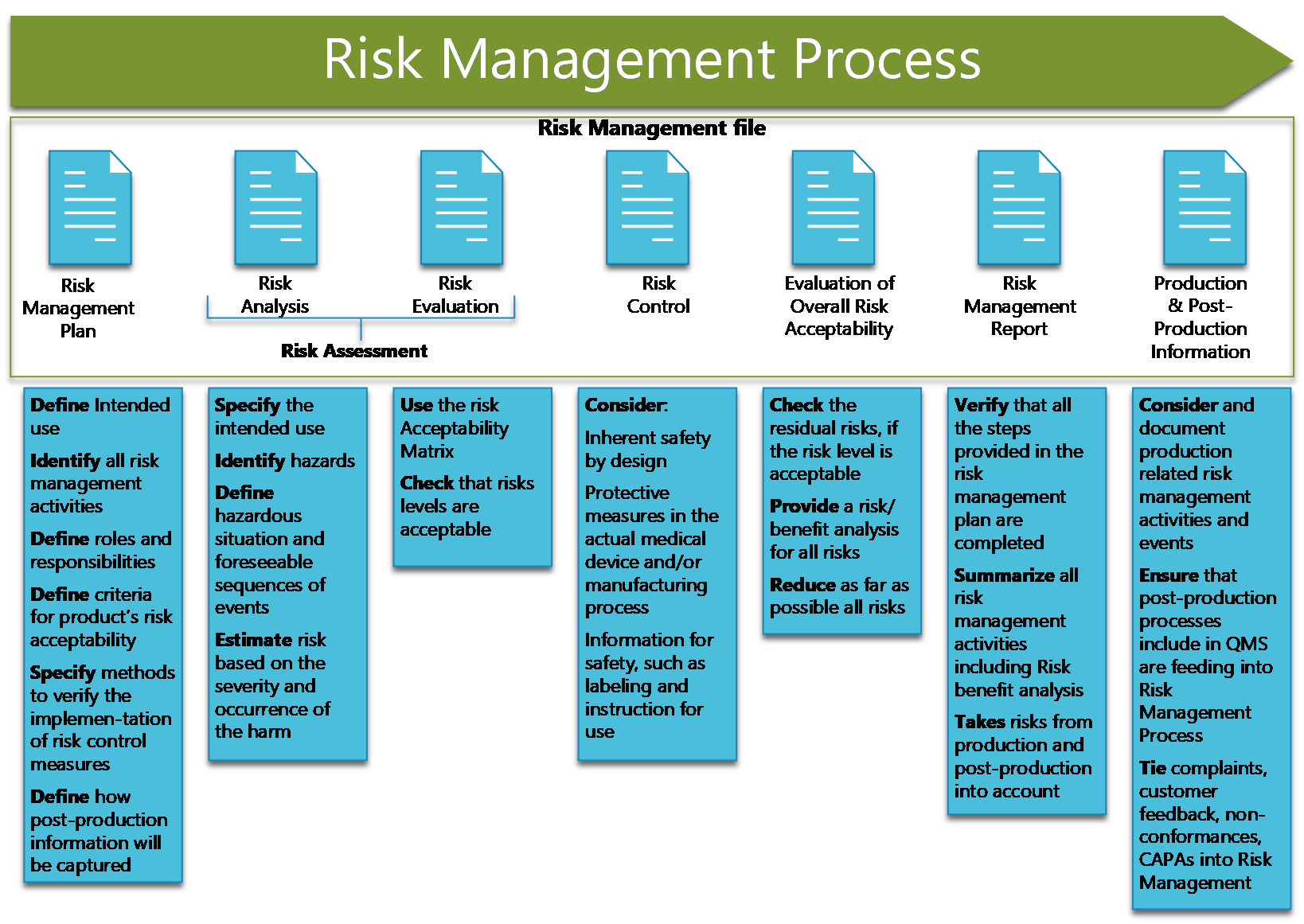
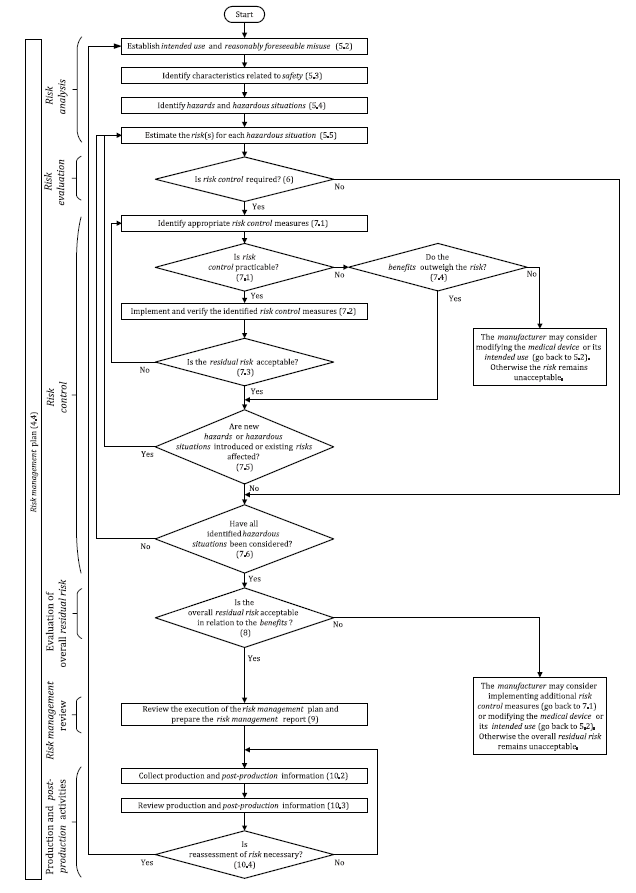
Iso 14971 Template
Iso 14971 Template - Web the requirements of this document are applicable to all phases of the life cycle of a medical device. Web download free template. Web risk management plan template , which can be used as starting point for the practical implementation of the risk management process; Your risk management plan must include: Learn what is expected from regulators & how to leverage risk as a tool. This includes software as a medical device. Web preview this document specifies terminology, principles and a process for risk management of medical devices, including software as a medical device and in vitro. Web the definitive guide to iso 14971 risk management for medical devices. Web as such, there is specific documentation for alignment with iso 14971. Web the third edition of iso 14971 was published in december 2019 and supersedes the second edition of iso 14971.
Iso 14971 Risk Management Plan Example
Oliver eidel template download this is a free template, provided by. Web the requirements of this document are applicable to all phases of the life cycle of a medical device. Your risk management plan must include: Web preview this document specifies terminology, principles and a process for risk management of medical devices, including software as a medical device and in.
ISO 149712019 ( Medical Device Risk management ) Detailed
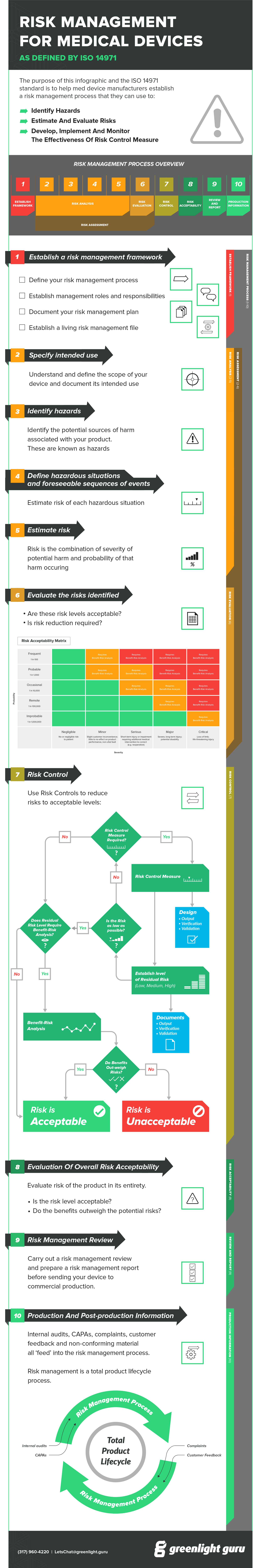
Web here at qualitymeddev we have a risk management plan template fully editable in word that can be used as starting point for the construction of your risk. Your risk management plan must include: Web key definitions implementing iso 14971 initiating risk management and design controls part 1: Risk management plan risk acceptability criteria residual risk. Web as such, there.
ISO 14971 Risk Management for Medical Devices The Definitive Guide
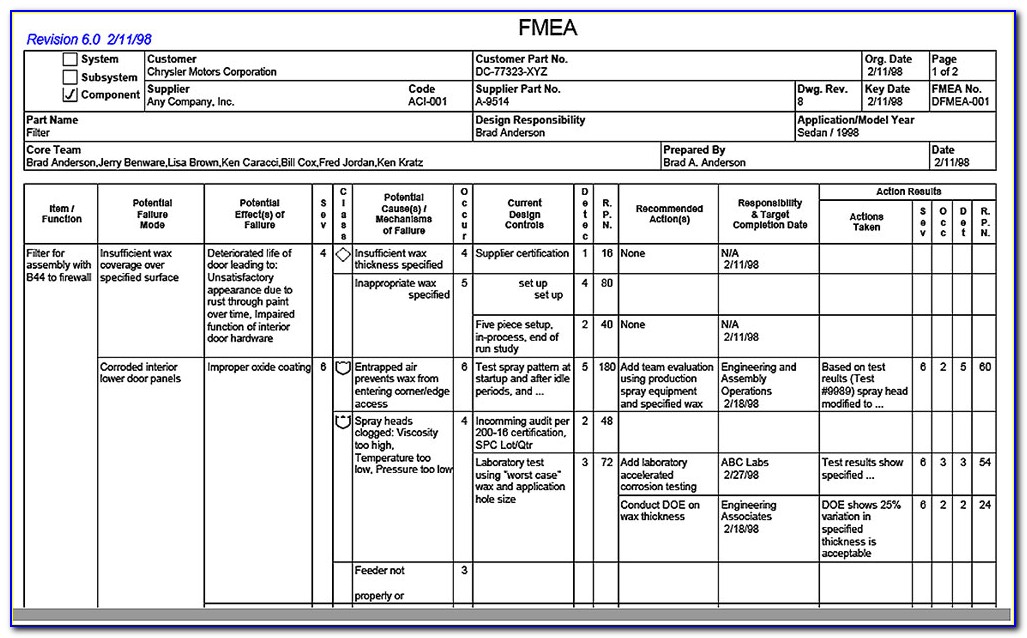
Your risk management plan must include: Web standard operating procedure (sop) for risk management according to en iso 14971:2019. Web iso 14971:2019, fmea, and risk management the separation of fmea from the risk management process is important because the goals of each of these activities. Our templates currently cover compliance for iso 13485, iec 62304, iso 14971 and iec 62366..
Risk analysis iso 14971 template mokasincave
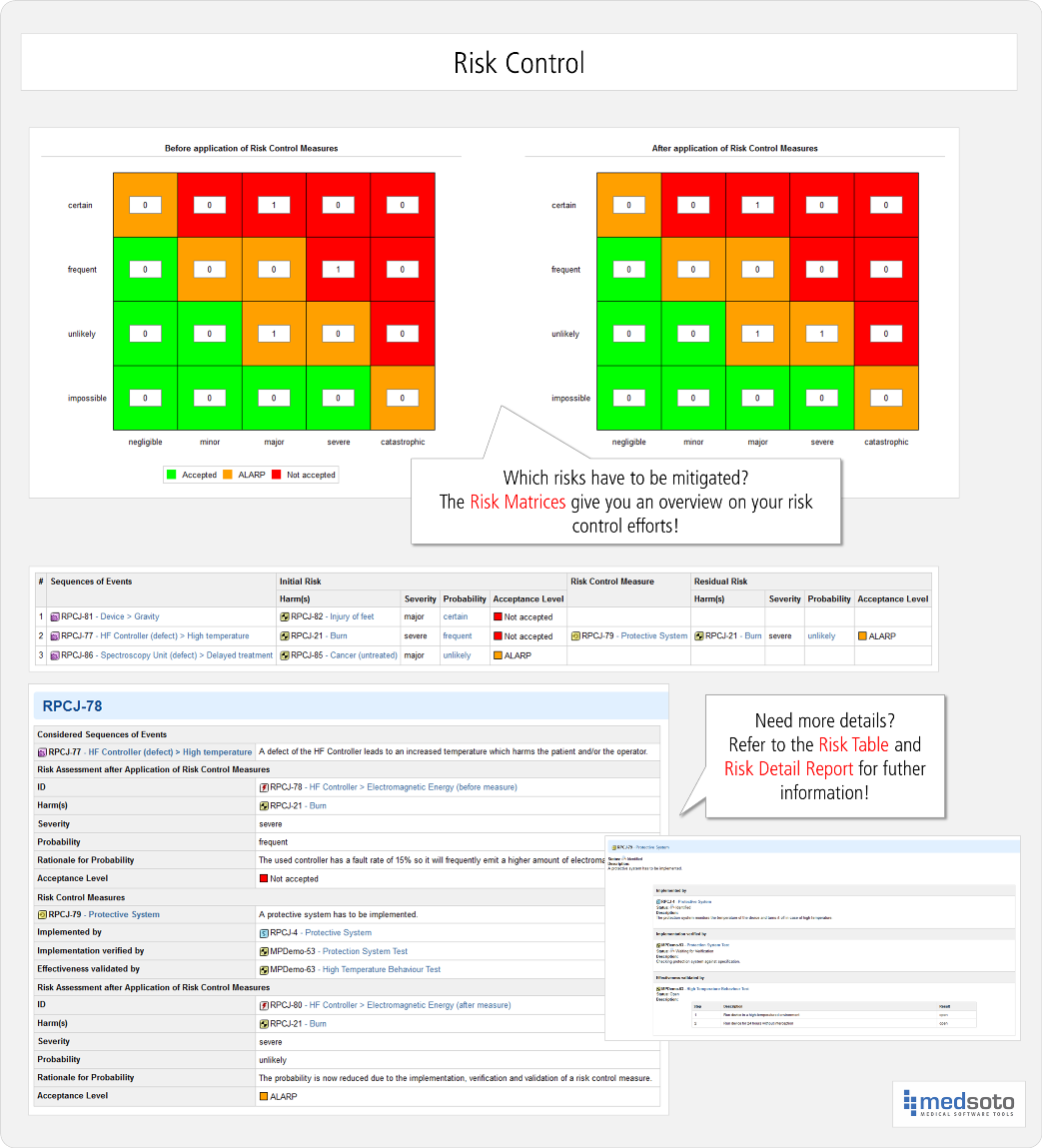
Web download free template. In the beginning, there’s a hazard, like a. Web risk management plan template , which can be used as starting point for the practical implementation of the risk management process; Web the 14971 wants you to analyze hazards, hazardous situations and harms, so that’s what you’ll find in the table :) here’s what happens: Web here.
Iso14971 Risk Management Template Third edition of ISO 14971
In the beginning, there’s a hazard, like a. Oliver eidel iso 14971 is the standard for risk management of medical device software. Your risk management plan must include: Risk management plan risk acceptability criteria residual risk. Web international standard bs en iso 14971 [1] was developed to provide a process to assist manufacturers in identifying the hazards associated with medical.
Risk analysis iso 14971 template systemmokasin
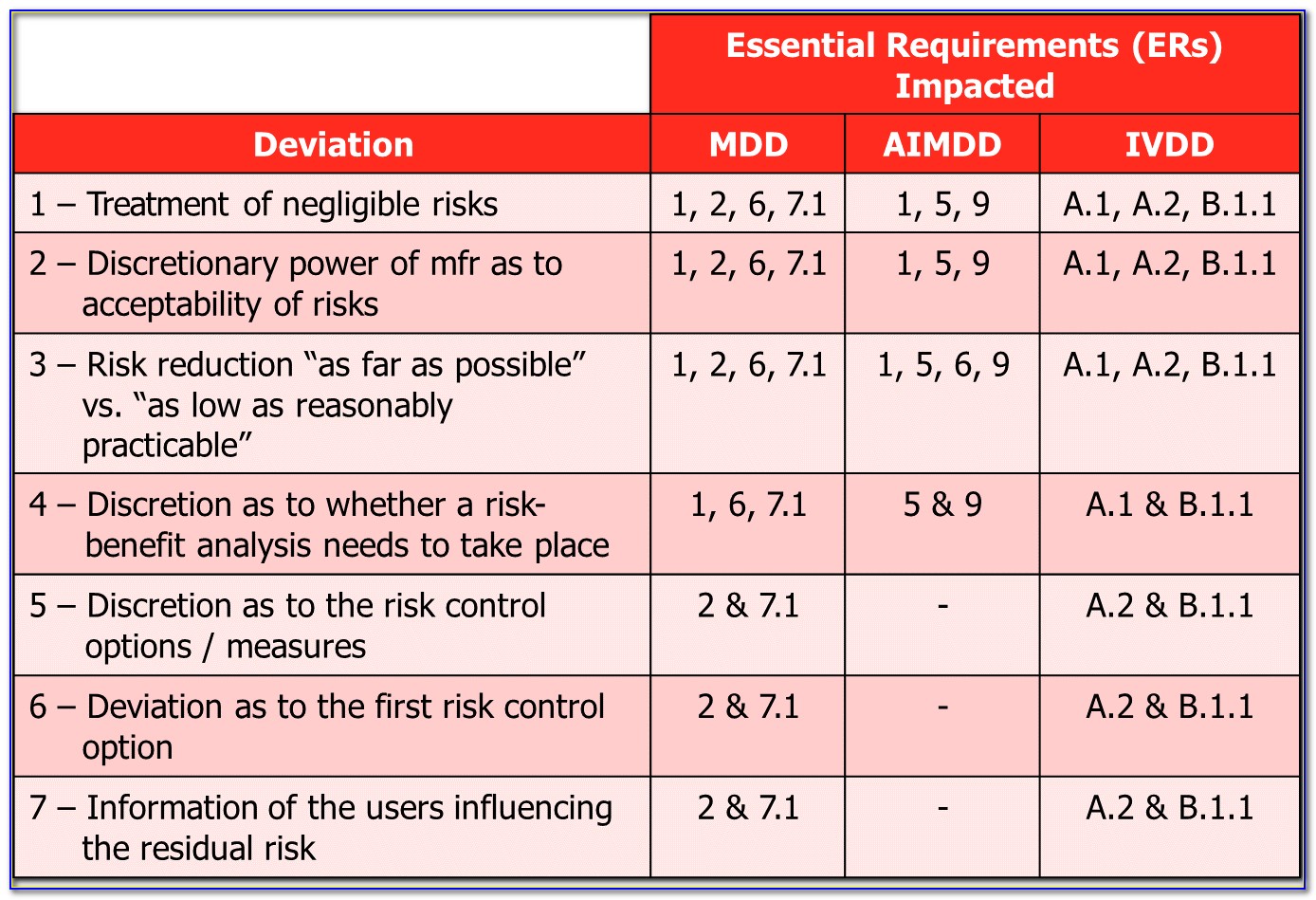
Web international standard bs en iso 14971 [1] was developed to provide a process to assist manufacturers in identifying the hazards associated with medical devices, assessing the. This includes software as a medical device. Web preview this document specifies terminology, principles and a process for risk management of medical devices, including software as a medical device and in vitro. Oliver.
Iso 14971 Risk Management Plan Template
This includes software as a medical device. Web the definitive guide to iso 14971 risk management for medical devices. Your risk management plan must include: Oliver eidel iso 14971 is the standard for risk management of medical device software. Web the 14971 wants you to analyze hazards, hazardous situations and harms, so that’s what you’ll find in the table :).
ISO 14971 Risk Management Guidance Document Rev 1 (2016!06!27) PDF
This includes software as a medical device. Scope of the planned risk management activities. Web international standard bs en iso 14971 [1] was developed to provide a process to assist manufacturers in identifying the hazards associated with medical devices, assessing the. Web download them for free and get your compliance done, no strings attached. In the beginning, there’s a hazard,.
Risk Management for Medical Devices ISO 149712019 Kvalito
Web the definitive guide to iso 14971 risk management for medical devices. Iso 14971 is the risk management standard for medical devices. The process described in this document applies to risks associated with a. Scope of the planned risk management activities. Web the requirements of this document are applicable to all phases of the life cycle of a medical device.
ISO 14971 Risk Management for medical devices Kobridge
Web international standard bs en iso 14971 [1] was developed to provide a process to assist manufacturers in identifying the hazards associated with medical devices, assessing the. This includes software as a medical device. Ad international organization for standards. Web key definitions implementing iso 14971 initiating risk management and design controls part 1: Oliver eidel template download this is a.
Our templates currently cover compliance for iso 13485, iec 62304, iso 14971 and iec 62366. Web risk management plan template , which can be used as starting point for the practical implementation of the risk management process; Web iso 14971:2019, fmea, and risk management the separation of fmea from the risk management process is important because the goals of each of these activities. Web the requirements of this document are applicable to all phases of the life cycle of a medical device. Your risk management plan must include: This includes software as a medical device. Ad international organization for standards. Web standard operating procedure (sop) for risk management according to en iso 14971:2019. Web updated november 22, 2022 iso 14971 templates dr. Web templates iso 14971 templates updated june 27, 2022 template: Web the 14971 wants you to analyze hazards, hazardous situations and harms, so that’s what you’ll find in the table :) here’s what happens: Web here at qualitymeddev we have a risk management plan template fully editable in word that can be used as starting point for the construction of your risk. Web preview this document specifies terminology, principles and a process for risk management of medical devices, including software as a medical device and in vitro. In the beginning, there’s a hazard, like a. Scope of the planned risk management activities. Learn what is expected from regulators & how to leverage risk as a tool. Risk management plan risk acceptability criteria residual risk. The process described in this document applies to risks associated with a. Oliver eidel iso 14971 is the standard for risk management of medical device software. Web international standard bs en iso 14971 [1] was developed to provide a process to assist manufacturers in identifying the hazards associated with medical devices, assessing the.