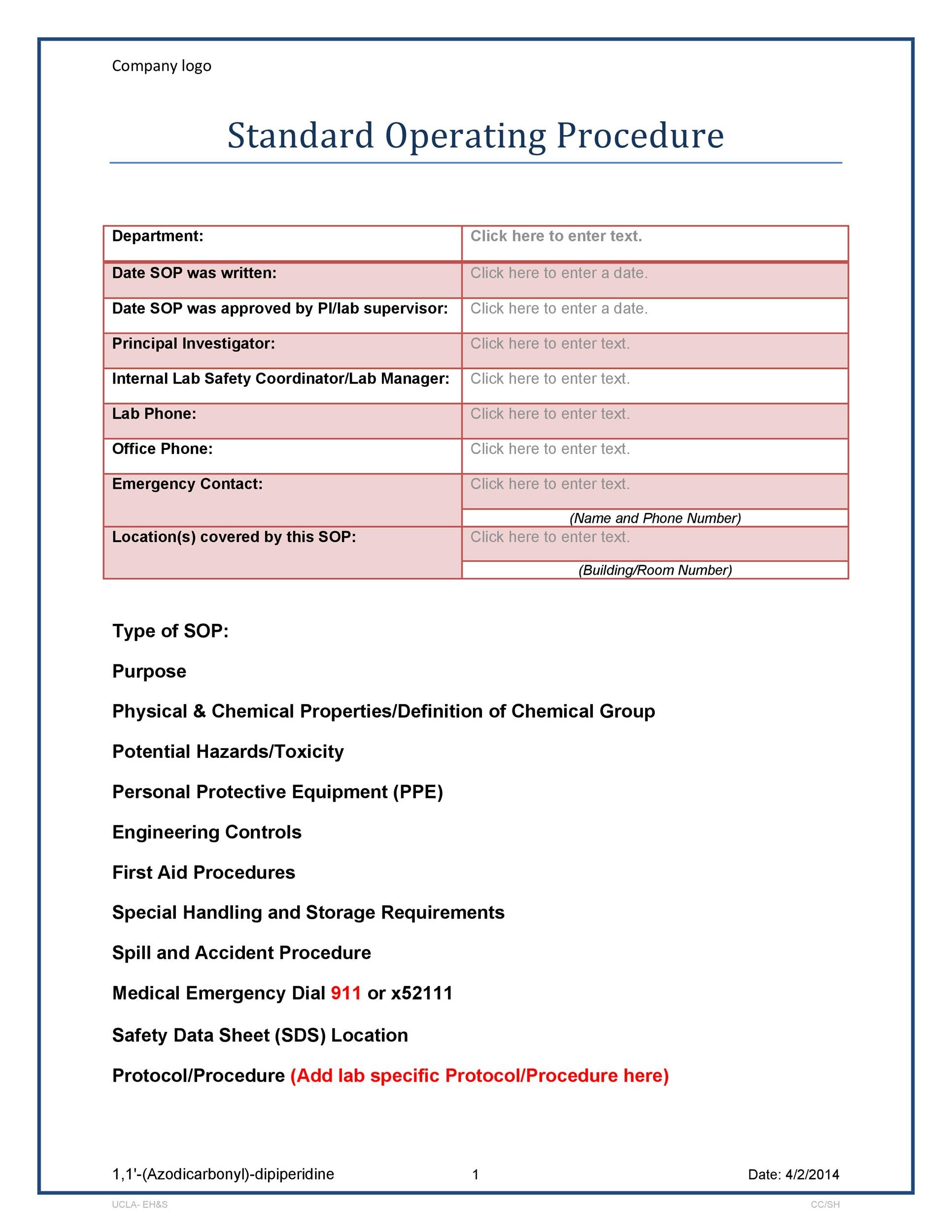
Medical Device Sop Templates
Medical Device Sop Templates - To name just a few, separate sops are required for each of the following: In total, we have 46+ procedures (listed below). Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them. The full set includes 105 sops,. Web clickup's medical device recall sop template is designed to help you efficiently manage and document the process of a medical device recall. Medical device standard operating procedure template group md500 consists of md50, md51,. Web it also includes procedures for canadian medical device licensing and european ce marking. These mdsap regulatory authority quality management system (qms) procedures provide a transparent overview on how. Transporting and storing products 4. Use these 8 templates to get started.
37 Best Standard Operating Procedure (SOP) Templates
This doc template contains all. Group md500 material controls sop templates. What our customers say about our sop templates!. These mdsap regulatory authority quality management system (qms) procedures provide a transparent overview on how. Web using one of our standard operating procedure template (sop template) will save you money and time due to quick and easy adaptation according to your.
DESIGN CONTROLS SOP Template MD20 GMP, QSR & ISO Compliance
Group md500 material controls sop templates. Web mdsap qms procedures and forms. Medical device standard operating procedure template group md500 consists of md50, md51,. Web standard operating procedure (sop) for risk management according to en iso 14971:2019 99 € (ex. The compliance monitoring team has created standard operating procedure templates (sops) in response to.
11 Editable Standard Operating Procedure Template SampleTemplatess
Web the fda qsr establishes the requirements for medical device quality systems, including requirements for medical device companies to develop and document standard. Web clickup's medical device recall sop template is designed to help you efficiently manage and document the process of a medical device recall. The full set includes 105 sops,. Group md500 material controls sop templates. Transporting and.
PROCESS VALIDATION SOP Template MD46 GMP, QSR & ISO Compliance
In total, we have 46+ procedures (listed below). Web iso 13485 template for medical devices. Web md30 corrective and preventive action sop templates. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them. Web standard operating procedure (sop) for risk management according to en iso 14971:2019.
MEDICAL DEVICE REPORTING SOP Template MD33 GMP, QSR & ISO Comp
What our customers say about our sop templates!. The full set includes 105 sops,. Web it also includes procedures for canadian medical device licensing and european ce marking. As a german manufacturer, you are also subject to national law. Web using one of our standard operating procedure template (sop template) will save you money and time due to quick and.
DESIGN HISTORY FILE SOP Template MD26 GMP, QSR & ISO Compliance
What our customers say about our sop templates!. Medical device standard operating procedure template group md500 consists of md50, md51,. Medical device companies must adhere to a lotof requirements to maintain regulatory compliance, and those must be clearly documented. Use these 8 templates to get started. To name just a few, separate sops are required for each of the following:
FREE 61+ SOP Templates in PDF MS Word
The full set includes 105 sops,. Use these 8 templates to get started. Medical device standard operating procedure template group md500 consists of md50, md51,. Web using one of our standard operating procedure template (sop template) will save you money and time due to quick and easy adaptation according to your needs. Web this sop describes the development of medical.
9+ Standard Operating Procedure (SOP) Templates Word Excel PDF Formats
Transporting and storing products 4. Web using one of our standard operating procedure template (sop template) will save you money and time due to quick and easy adaptation according to your needs. Web iso 13485 template for medical devices. Web the fda qsr establishes the requirements for medical device quality systems, including requirements for medical device companies to develop and.
SOP Center EMS Template
Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them. Transporting and storing products 4. Web using one of our standard operating procedure template (sop template) will save you money and time due to quick and easy adaptation according to your needs. Web it also includes.
QUALITY INVESTIGATIONS SOP Template MD35 GMP, QSR & ISO Comp
Medical device companies must adhere to a lotof requirements to maintain regulatory compliance, and those must be clearly documented. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them. The full set includes 105 sops,. In total, we have 46+ procedures (listed below). To name just.
As a german manufacturer, you are also subject to national law. Web standard operating procedure (sop) for risk management according to en iso 14971:2019 99 € (ex. Web it also includes procedures for canadian medical device licensing and european ce marking. Group md500 material controls sop templates. Web the fda qsr establishes the requirements for medical device quality systems, including requirements for medical device companies to develop and document standard. Web clickup's medical device recall sop template is designed to help you efficiently manage and document the process of a medical device recall. What our customers say about our sop templates!. Web using one of our standard operating procedure template (sop template) will save you money and time due to quick and easy adaptation according to your needs. Medical device companies must adhere to a lotof requirements to maintain regulatory compliance, and those must be clearly documented. Medical device standard operating procedure template group md500 consists of md50, md51,. To name just a few, separate sops are required for each of the following: Transporting and storing products 4. These mdsap regulatory authority quality management system (qms) procedures provide a transparent overview on how. The full set includes 105 sops,. In total, we have 46+ procedures (listed below). The compliance monitoring team has created standard operating procedure templates (sops) in response to. This doc template contains all. Web md30 corrective and preventive action sop templates. Web this sop describes the development of medical devices in accordance with regulatory requirements of annex xiv (mdr) regarding medical device clinical performance. Web iso 13485 template for medical devices.