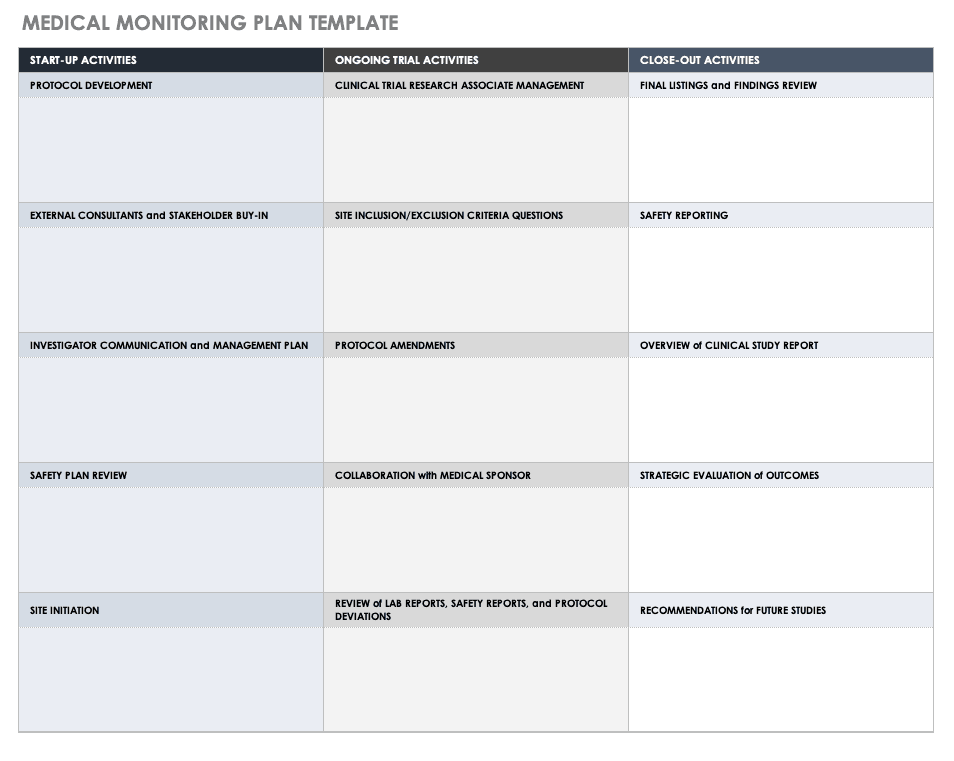
Medical Monitoring Plan Template
Medical Monitoring Plan Template - Web nidcr clinical monitoring guidelines. Web nimh clinical monitoring plan template [word] this template provides a recommended structure for a plan to conduct internal or independent review of good clinical practices. Best practice recommendations • review this draft. Web clinical study report template. Web this webpage provides guidance for niddk grant applicants on. Find out how our patient monitoring products can help deliver operational efficiencies. Web 1.1 purpose the california code of regulations, title 8 requires that employees of california state university, chico (the “university”) with exposure potential to certain. Also, we have included a proposed structure for a. Throughout the template there are suggested. Exampledata and safety monitoring plan (dsmp)independent monitor.
3.8. Template for monitoring plan
Which clinical studies require a. Exampledata and safety monitoring plan (dsmp)independent monitor. Web designated medical monitor. Also, we have included a proposed structure for a. Web guidelines for developing a data and safety monitoring plan.
Care Physical health International Cat Care
Also, we have included a proposed structure for a. Guidance for clinical research associates responsible for preparing a clinical monitoring plan. Exampledata and safety monitoring plan (dsmp)independent monitor. Web clinical monitoring plan template. Find out how our patient monitoring products can help deliver operational efficiencies.
hospital compliance plan Jennies Blog hospital risk management
Web 1.1 purpose the california code of regulations, title 8 requires that employees of california state university, chico (the “university”) with exposure potential to certain. Web this is an ms word template to use as a starting point for preparing a medical monitoring plan for clinical trials or research. Also, we have included a proposed structure for a. Web purpose.
Patient Tracking Spreadsheet —
Guidance for assisting grantees conducting or planning to conduct clinical trials, has developed these. Throughout the template there are suggested. Who needs a data and safety monitoring plan (dsmp) the content of a dsmp. Guidance document that provides detailed descriptions of the nidcr clinical monitoring processes. Web the national institute of mental health (nimh) has developed the following guidance for.
Monitoring Plan Template
Find out how our patient monitoring products can help deliver operational efficiencies. Web monitoring plan template page 1of 17 version date: Throughout the template there are suggested. Web clinical study report template. Best practice recommendations • review this draft.
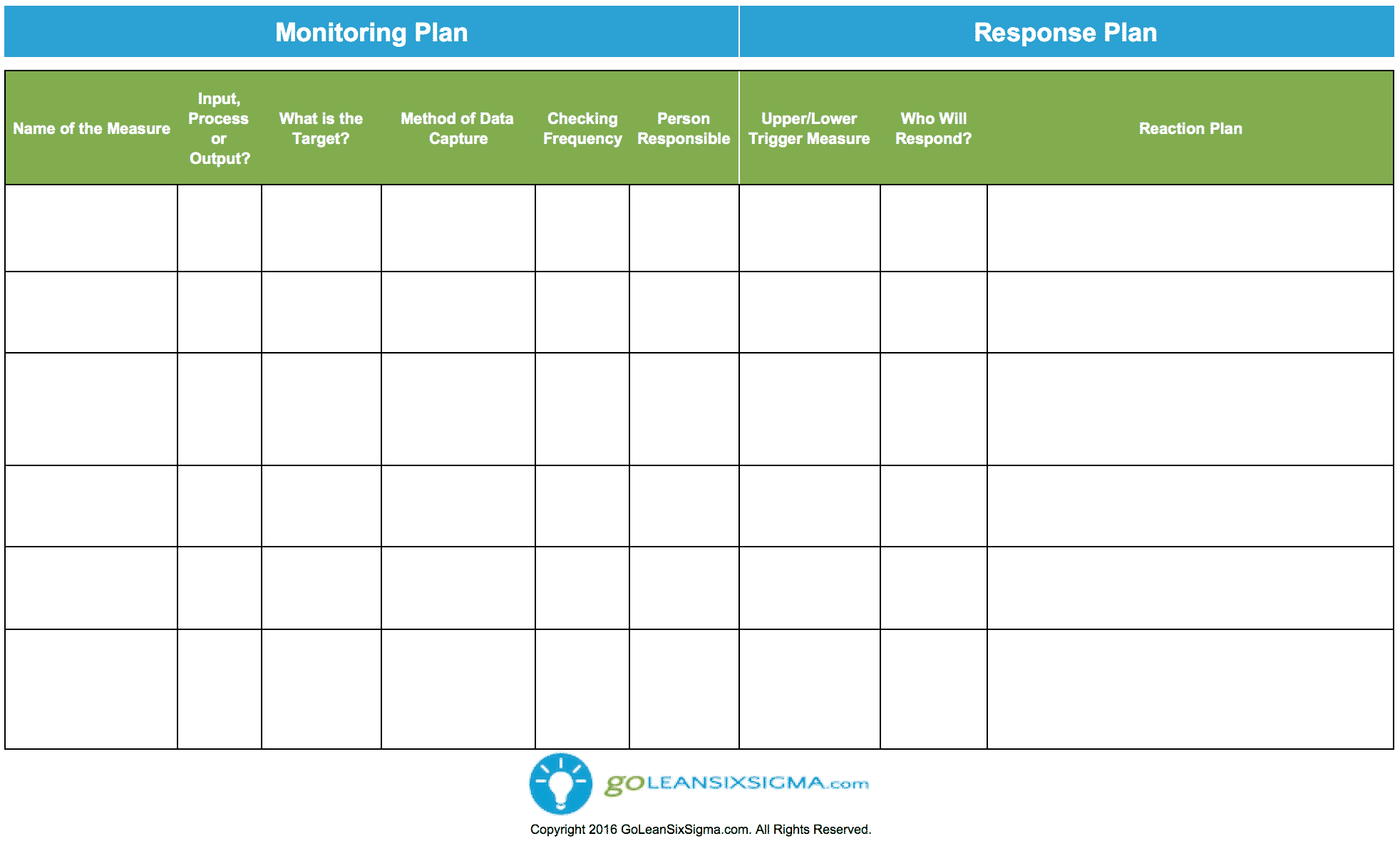
Monitoring & Response Plan Template & Example
Find out how our patient monitoring products can help deliver operational efficiencies. Web this template includes a proposed structure for a clinical monitoring plan as well as draft language and other guidance. Guidance for assisting grantees conducting or planning to conduct clinical trials, has developed these. Exampledata and safety monitoring plan (dsmp)independent monitor. Web this is an ms word template.
The enchanting The Basics Of Clinical Trial Centralized Monitoring For
Web monitoring plan template page 1of 17 version date: Ad learn how philips patient monitoring can help unlock capabilities across your system. Exampledata and safety monitoring plan (dsmp)independent monitor. Web this webpage provides guidance for niddk grant applicants on. Who needs a data and safety monitoring plan (dsmp) the content of a dsmp.
Example of Monitoring Sheet PDF Teachers Curriculum
14 march 2019page 1 of 17 n2 quality committee guidance for developing monitoring plan s introduction the. Web this is an ms word template to use as a starting point for preparing a medical monitoring plan for clinical trials or research. Web designated medical monitor. Which clinical studies require a. Web this template includes a proposed structure for a clinical.
Free Medical Form Templates Smartsheet
Web this template includes a proposed structure for a clinical monitoring plan as well as draft language and other guidance. Ad learn how philips patient monitoring can help unlock capabilities across your system. 14 march 2019page 1 of 17 n2 quality committee guidance for developing monitoring plan s introduction the. Find out how our patient monitoring products can help deliver.
Monitoring Report Template Clinical Trials ] Saving Lives Pertaining
Guidance for assisting grantees conducting or planning to conduct clinical trials, has developed these. Throughout the template there are suggested. Best practice recommendations • review this draft. Web nimh clinical monitoring plan template [word] this template provides a recommended structure for a plan to conduct internal or independent review of good clinical practices. Web clinical study report template.
Web purpose {explain purpose of monitoring plan, for example:} the purpose of this monitoring plan is to describe the rationale and process for the collection, recording,. Web this is an ms word template to use as a starting point for preparing a medical monitoring plan for clinical trials or research. Web guidelines for developing a data and safety monitoring plan. Guidance for assisting grantees conducting or planning to conduct clinical trials, has developed these. Find out how our patient monitoring products can help deliver operational efficiencies. Exampledata and safety monitoring plan (dsmp)independent monitor. Web investigators should consider using this template when developing the data and safety monitoring plan (dsmp) for clinical studies funded by the national institute of arthritis. Web nidcr clinical monitoring guidelines. Web this webpage provides guidance for niddk grant applicants on. Web example data and safety monitoring plan (dsmp) independent monitor. Web 1.1 purpose the california code of regulations, title 8 requires that employees of california state university, chico (the “university”) with exposure potential to certain. Who needs a data and safety monitoring plan (dsmp) the content of a dsmp. Web monitoring plan template page 1of 17 version date: Web clinical study report template. Best practice recommendations • review this draft. Also, we have included a proposed structure for a. Ad learn how philips patient monitoring can help unlock capabilities across your system. Web the national institute of mental health (nimh) has developed the following guidance for investigators developing a data and safety monitoring plan (dsmp) to. Web clinical monitoring plan template. Guidance document that provides detailed descriptions of the nidcr clinical monitoring processes.






![Monitoring Report Template Clinical Trials ] Saving Lives Pertaining](https://pray.gelorailmu.com/wp-content/uploads/2020/01/monitoring-report-template-clinical-trials-saving-lives-pertaining-to-monitoring-report-template-clinical-trials-1603x2048.png)