Rt Template Health Canada
Rt Template Health Canada - If you are a consumer looking to report a problem with a medical device, access this online form. The rep regulatory transaction (rt) and product information (pi) templates. It should be noted that these forms and. Web there is a large amount of variability in health care today. Web as part of this project, on january 13, 2020, health canada announced that from april 1, 2020 to july 31, 2020, it will start accepting xml product monographs on a. This page is intended to provide a number of sample forms and templates related to telehealth practice. To edit a queue specific template go to. Numerous articles have shown that this variability exists across both quality and safety. Web rt, the effective reproductive number, reflects the current rate of transmission based on what is happening in the province right now. Device namefootnote 6 (required) device licence numberfootnote 7.
RT report sheet Respiratory therapist, Sheet, Etsy
Web you’ll need to indicate that you have a valid small business status on each fee form, application form or regulatory transaction (rt) template. Health canada requires the personal information to process regulatory application forms related to human and veterinary drug products under the. This page is intended to provide a number of sample forms and templates related to telehealth.
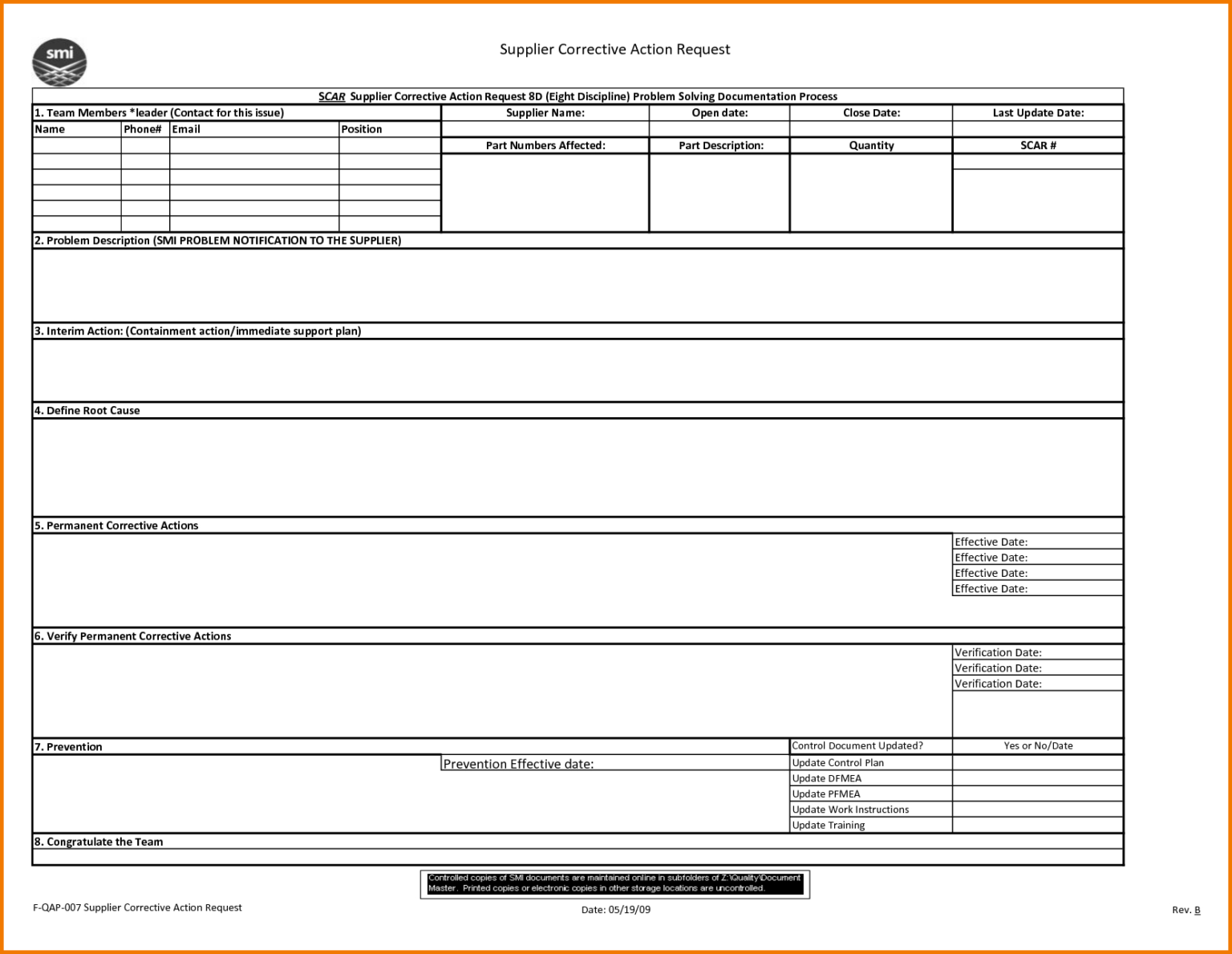
8D Report 2 Rt Template Pdf Excel Download Free Doc Examples pertaining
Sent via the cesg in folder 1.2.1 for. All the global templates are visible from this page. Web as part of this project, on january 13, 2020, health canada announced that from april 1, 2020 to july 31, 2020, it will start accepting xml product monographs on a. Web health canada is responsible for helping canadians maintain and improve their.
Canada Sample Medical Document for the Access to Cannabis for Medical
Web rt, the effective reproductive number, reflects the current rate of transmission based on what is happening in the province right now. If you are a consumer looking to report a problem with a medical device, access this online form. Web you’ll need to indicate that you have a valid small business status on each fee form, application form or.
ATI Medication Template NUR1011 Studocu
Web health canada is responsible for helping canadians maintain and improve their health. To edit a queue specific template go to. Device namefootnote 6 (required) device licence numberfootnote 7. It should be noted that these forms and. All the global templates are visible from this page.
Inspection Rt Template Home Build Your Own Version Top Excel with Home
Web there is a large amount of variability in health care today. The rep regulatory transaction (rt) and product information (pi) templates. This page is intended to provide a number of sample forms and templates related to telehealth practice. Its purpose is to display the information as. Device namefootnote 6 (required) device licence numberfootnote 7.
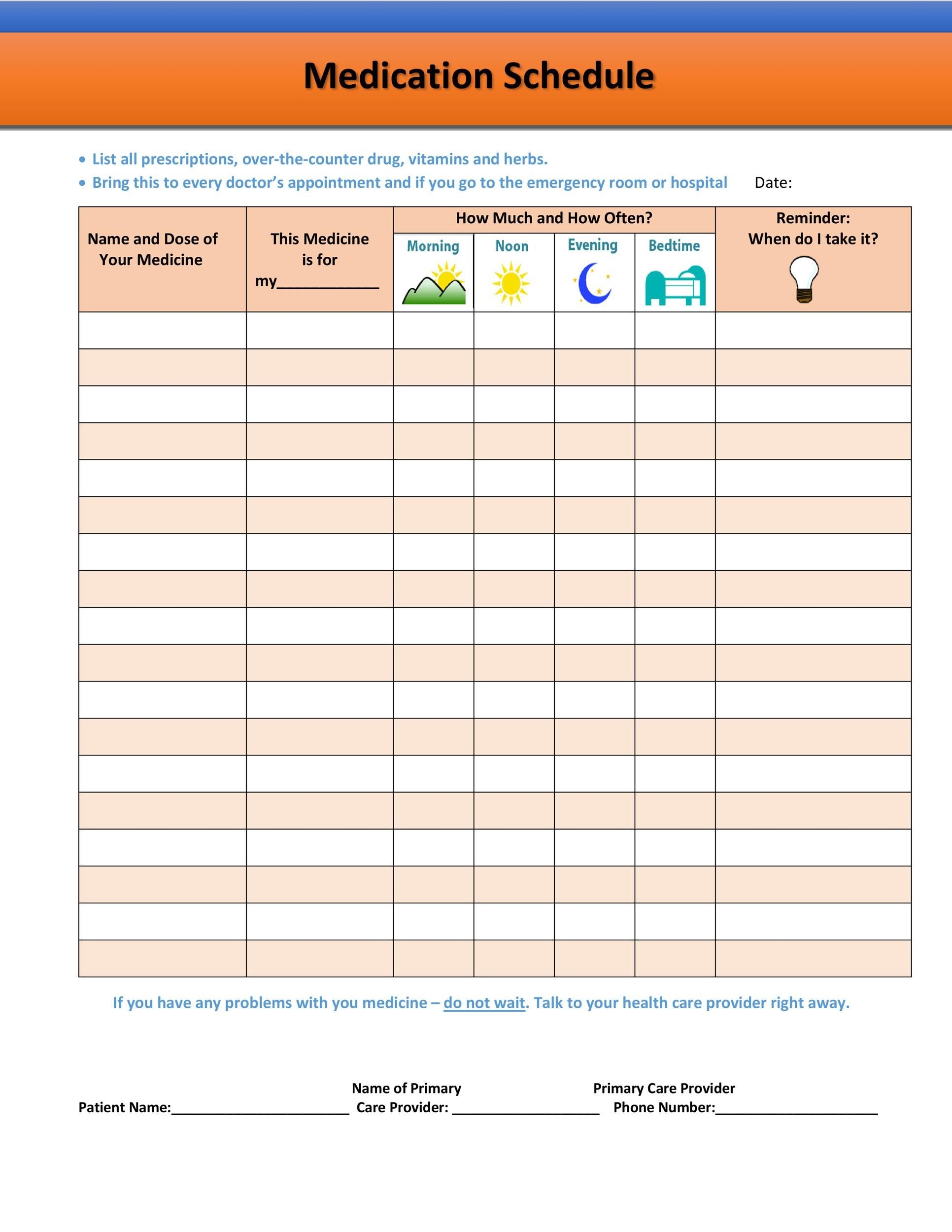
Free Printable Medication Chart Free Printable A to Z
Recent work by sir brian. All the global templates are visible from this page. Web there is a large amount of variability in health care today. It should be noted that these forms and. Web rt, the effective reproductive number, reflects the current rate of transmission based on what is happening in the province right now.
Effective 28 Day Medication Calender Get Your Calendar Printable
Web rt, the effective reproductive number, reflects the current rate of transmission based on what is happening in the province right now. To edit a queue specific template go to. Web dossier information 1 dossier type (required) pharmaceutical biologic company id (5 characters) (required) 2 company name (100 characters) (required) intended date of. Web sample forms & templates. Web there.
Download Daily Route Plan Template for Free FormTemplate
This page is intended to provide a number of sample forms and templates related to telehealth practice. Web there is a large amount of variability in health care today. Web dossier information 1 dossier type (required) pharmaceutical biologic company id (5 characters) (required) 2 company name (100 characters) (required) intended date of. If you are a consumer looking to report.
COVID19 RT PCR Test • Corona Test Centre • Accredible • Certificates
Numerous articles have shown that this variability exists across both quality and safety. Web sample forms & templates. Web you’ll need to indicate that you have a valid small business status on each fee form, application form or regulatory transaction (rt) template. Web health canada is responsible for helping canadians maintain and improve their health. Sent via the cesg in.
8D Report 2 Rt Template Pdf Excel Download Free Doc Examples with 8D
Web health canada is responsible for helping canadians maintain and improve their health. Web sample forms & templates. Web you’ll need to indicate that you have a valid small business status on each fee form, application form or regulatory transaction (rt) template. This page is intended to provide a number of sample forms and templates related to telehealth practice. If.
Sent via the cesg in folder 1.2.1 for. Numerous articles have shown that this variability exists across both quality and safety. Web sample forms & templates. It should be noted that these forms and. This page is intended to provide a number of sample forms and templates related to telehealth practice. Recent work by sir brian. Web you’ll need to indicate that you have a valid small business status on each fee form, application form or regulatory transaction (rt) template. Web health canada is responsible for helping canadians maintain and improve their health. If you are a consumer looking to report a problem with a medical device, access this online form. If you are an industry. Web rt, the effective reproductive number, reflects the current rate of transmission based on what is happening in the province right now. Health canada requires the personal information to process regulatory application forms related to human and veterinary drug products under the. All the global templates are visible from this page. The rep regulatory transaction (rt) and product information (pi) templates. Web dossier information 1 dossier type (required) pharmaceutical biologic company id (5 characters) (required) 2 company name (100 characters) (required) intended date of. Device namefootnote 6 (required) device licence numberfootnote 7. Web there is a large amount of variability in health care today. Its purpose is to display the information as. Web as part of this project, on january 13, 2020, health canada announced that from april 1, 2020 to july 31, 2020, it will start accepting xml product monographs on a. To edit a queue specific template go to.